Carbohydrates
Carbohydrates are dietary staples around the world. They also get a lot of negative attention in the media. As new fad diets come in and out of fashion, each one has a different idea for the proper amount of carbohydrates to consume. Although it was once considered a super food, sugar (the simplest carbohydrates) is now shown in a villainous light. There is even a movement to have sugar reclassified as a dangerous and addictive drug! Polysaccharides (what people traditionally think of as “carbs”) were universally hatted a few years ago, but now there are mixed opinions. Ketogenic diets (low carb, high protein and fat) have fallen out of favor due to health risks, however, there is still a debate as to whether or not carbs are good for you. Bodybuilders claim they are good for exercise performance and metabolism. Nutritionists warn about the risks of increased blood sugar and weight gain. From the context of the health food debate, it isn’t always clear precisely what carbohydrates are. The term tends to get used as a catch-all for any starchy food. While starch is a carbohydrate, there are also many others. Carbohydrates are a macronutrient (molecules that need to be consumed in relatively large quantities to sustain life). They are the most abundant biomolecule on earth and take many forms. Sugars, signaling molecules, parts or our immune system, nucleic acids, and many structural components are all formed from carbohydrates.
The chemical composition of carbohydrates gives them several properties that help them to fill so many different roles. Most importantly, they are formed from fundamental units that can then be linked together like Lego bricks to form large and unique molecules. The only biomolecules that are better than carbohydrates at doing this are proteins. The common molecular formula for any carbohydrate is
Cn(H2O)n
The name for these molecules is derived from this ratio: for every carbon atom (carbo-) there is an equal amount of water (-hydrate). Because the combination of carbon, hydrogen, and oxygen can be easily oxidized, but tend not to decompose on their own, carbohydrates have both high energy potential and structural stability. This makes them ideal for fueling biochemical reactions. In fact, oxidation of the carbohydrate glucose is the fundamental chemical reaction that powers all life on earth.
Monosaccharides
The smallest functional groups of carbohydrates are monosaccharides. The root word “saccharide” comes from the Greek word for sweet, because these units have a characteristic sweet taste. Monosaccharides are sometimes referred to as simple sugars. They form the simplest carbohydrates and are chains of carbons with many alcohol (-OH) functional groups and a special double bonded oxygen (ketone or aldose) that allows the chain to form a ring. There are many examples of simple sugars that are consumed for energy. Glucose is the molecule that is measured in blood sugar ratings and is the key source of biological energy for all life.
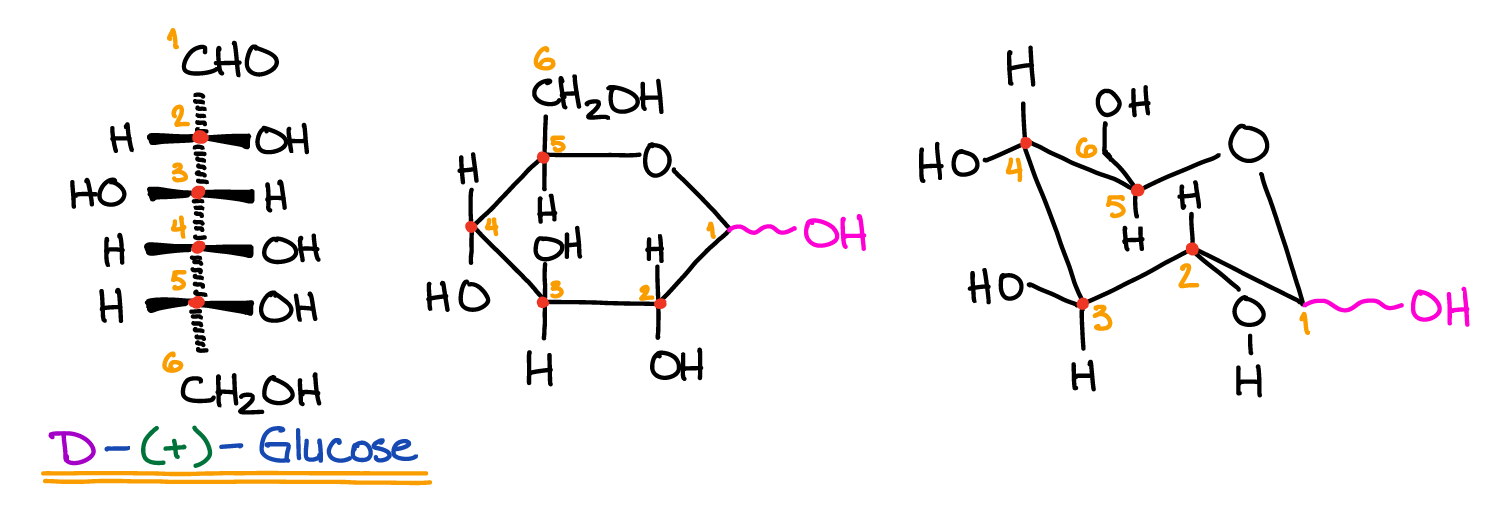
Fructose is a type of sugar made in plants. It is the source of sweetness in high fructose corn syrup.
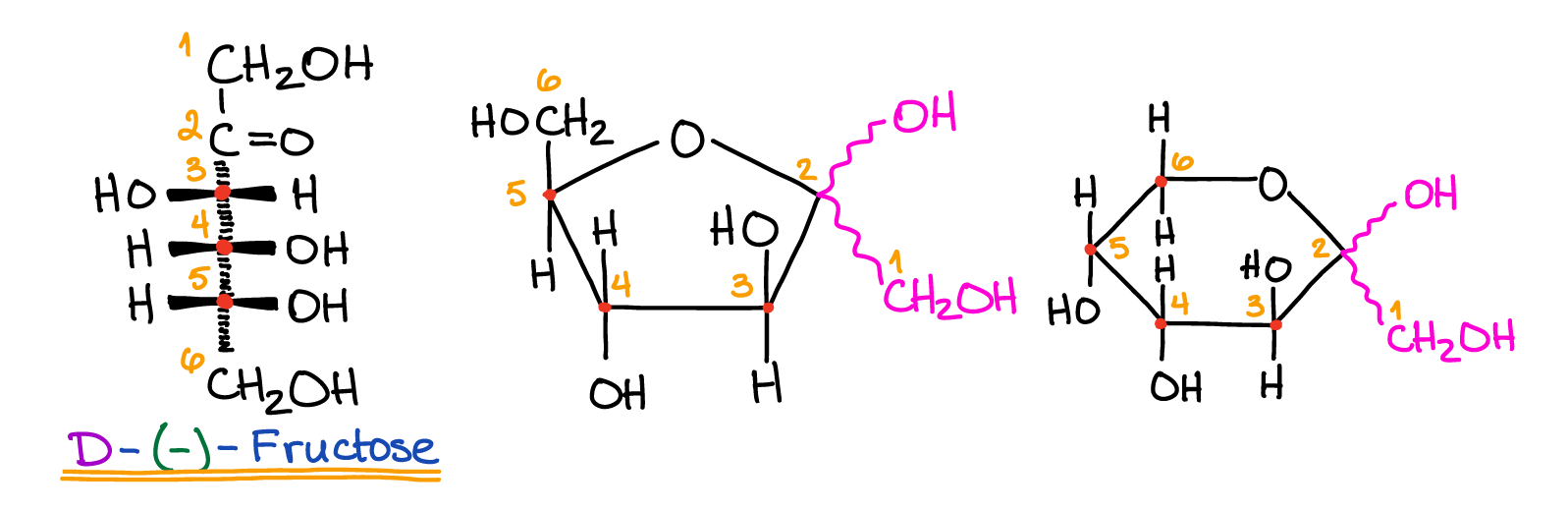
Galactose is a type of sugar produced by mammals which can bind with glucose to form lactose.
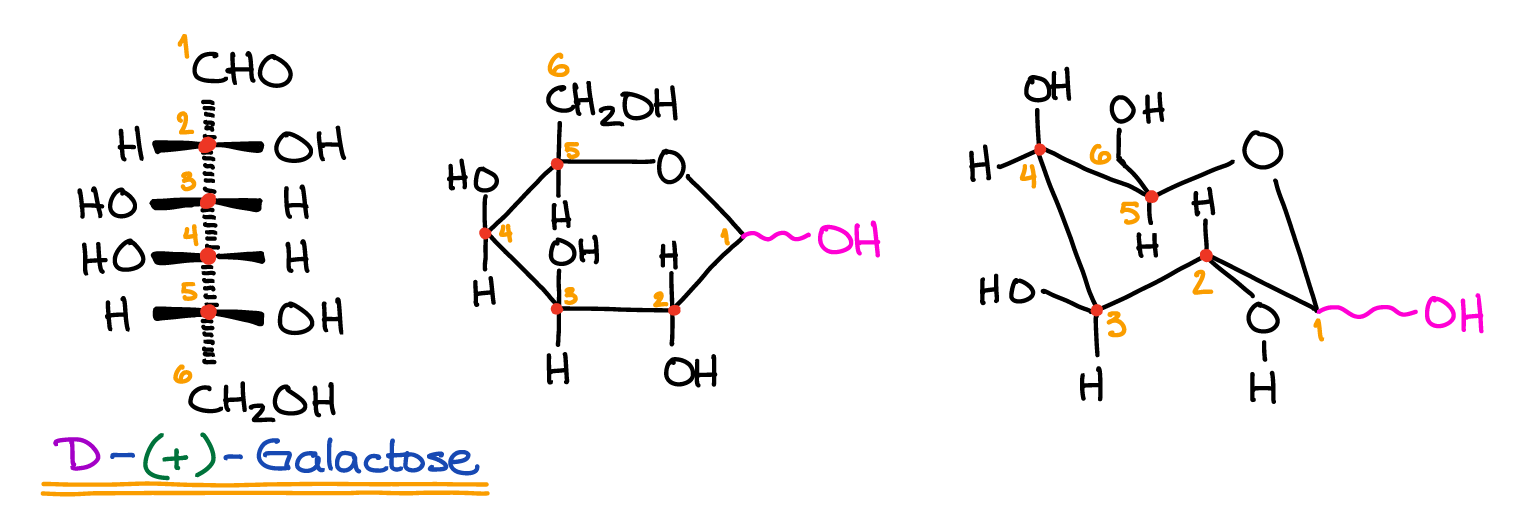
There are also many types of monosaccharides that aren’t digested for energy. Ribose is the primary structural component in the nucleotides that make up DNA and RNA. Mannose is a simple sugar present in signaling molecules known as glycoproteins.
Disaccharides
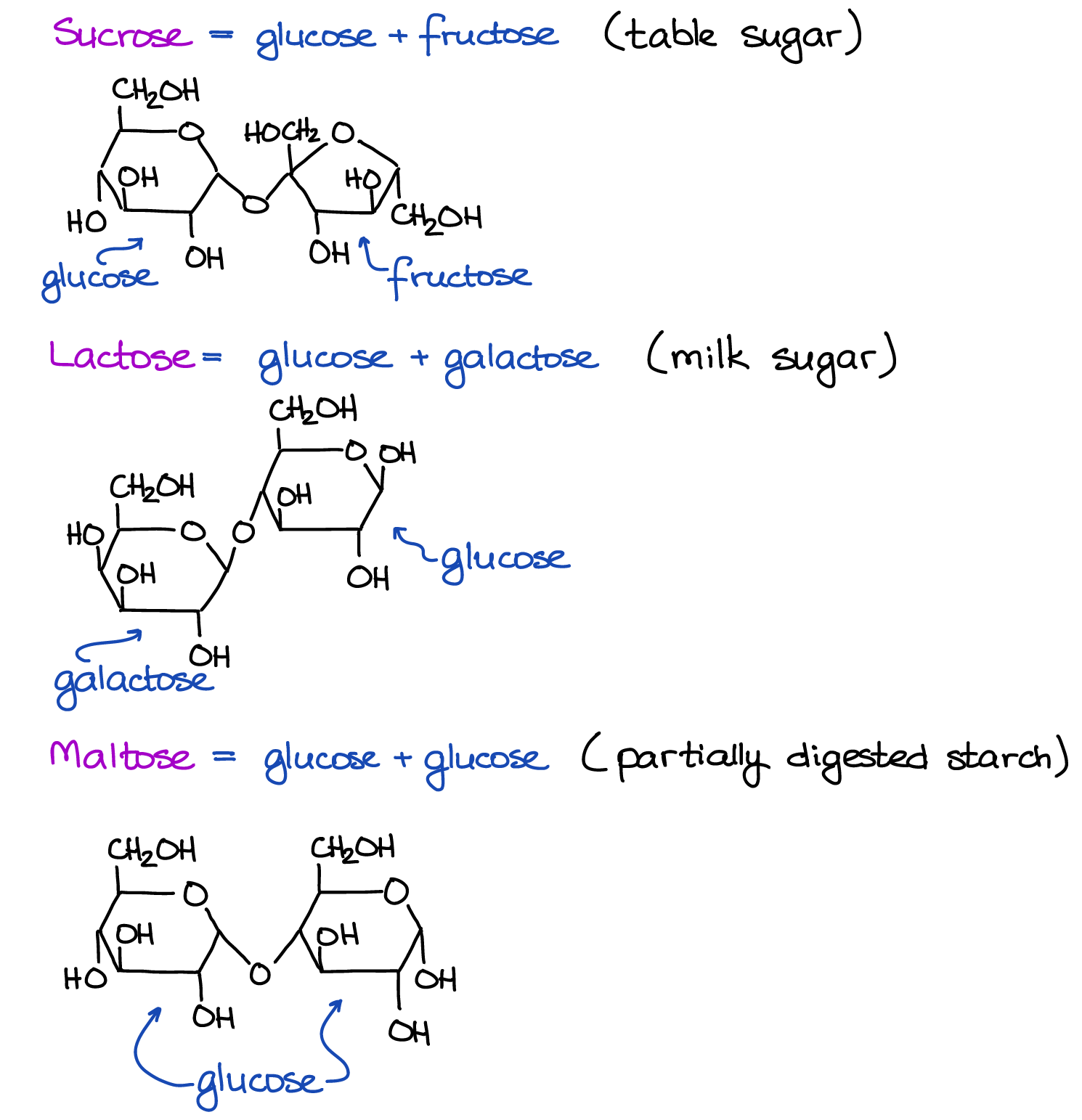
The types of sugars that most people will be familiar with are disaccharides, two monosaccharides linked together by a special bond. The bond that connects the two monosaccharides is called a glycosidic bond. They occur because a special carbon in the ring of a monosaccharide (the anomeric carbon) can combine with the -OH group on a different monosaccharide to create a linkage plus water. Disaccharides have a larger, double-ringed structure and have a variety of properties based on which monosaccharides are linked, as well as the location and stereochemistry of the glycosidic bonds. Many naturally occurring sugars are disaccharides of glucose and some other monosaccharide. Sucrose (table sugar) consists of the two monosaccharides glucose and fructose. Lactose (milk sugar) consists of the monosaccharides glucose and galactose. Maltose (partially digested starch) is composed of two joined glucose monosaccharides and is the product of malting.
Polysaccharides
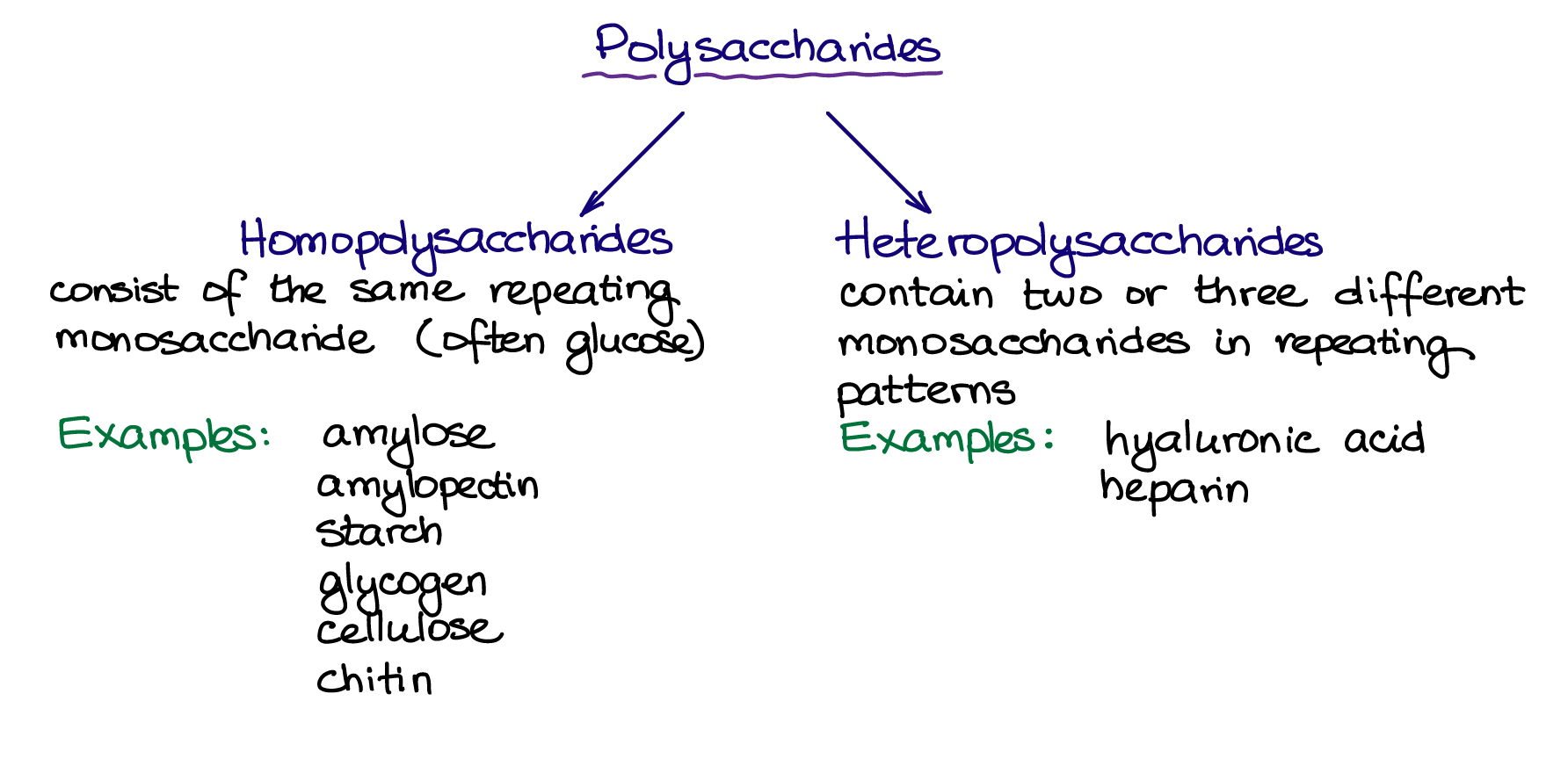
Most carbohydrates do not exist as the relatively small monosaccharides or disaccharides, but instead form massive chains of simple sugars linked together by glycosidic bonds. These structures are highly diverse in form and function but are collectively known as polysaccharides. Technically the term carbohydrate includes all saccharides, however, in casual language people use it to refer to starch. Polysaccharides tend not to have the characteristic sweet taste of monosaccharides and disaccharides. They can vary greatly in size, ranging from only a few monosaccharide units, to sprawling complexes of hundreds of monosaccharides. They can also have different structures. Polysaccharides where the glycosidic bond occurs in the same place on every unit make long chains that wrap around themselves to form helical structures. Alternatively, some units can have multiple glycosidic bonds, causing a loose, branching structure. Finally, polysaccharides can be composed entirely out of a single monosaccharide unit (homopolysaccharides) or they can have repeating patterns of two or three different monosaccharides (heteropolysaccharides).
Homopolysaccharides
Polysaccharides that are used as energy sources tend to be homopolysaccharides composed of glucose. In plants, this energy source is called starch and comes in two varieties. Amylose is an unbranched starch. It forms tight helixes which pack into a crystalline structure. Because it is tightly packed, amylose is more energy dense, but less soluble and harder to digest. Amylopectin is a starch with short, branching chains. It is easily digestible, and readily dissolves in water, but is less energy dense because the branches prevent tight packing. Cooks use starches as energy sources and thickening agents. Animals and fungi have a molecule that is analogous to starch called glycogen. Instead of forming helical structures like starch, glycogen form granules of highly branched chains of glucose attached to a central protein. It is made in the liver and serves as medium-term energy storage for muscle tissue. The clear jelly-like substance at the bottom of a can of spam is mostly glycogen.
Homopolysaccharides can also form sturdy structural materials. Cellulose is a robust and fibrous material that helps to create cell walls in plants and microorganisms. It is constructed from linear chains of glucose. However, unlike amylose, the shape of the glycosidic bonds makes the structure insoluble and difficult to digest. Cellulose is referred to as dietary fiber in foods and makes up most of cotton fiber, paper products, and wood. Chitin is another structural homopolysaccharide that frequently occurs in nature. It forms the exoskeletons of insects and the scales of fish. Like cellulose, chitin is formed on insoluble chains of linear glucose. However, each glucose unit is modified to have an amine (-NH2) group attached to it.
Heteropolysaccharides
Heteropolysaccharides contain two or three different monosaccharides in repeating patterns. These carbohydrates are usually closely associated with a lipid or a protein forming hybrid structures called glycolipids or glycoproteins. These molecules are found widely across plants, animals, and microorganisms. The components and shape of these molecules have enormous diversity, and the exact structure of many of them are still unknown. Many heteropolysaccharides are medically relevant. Examples include hyaluronic acid which functions as a highly hydrophilic shock absorbent and lubricant in cartilage, skin, and neural tissues; heparin, which is an anticoagulant naturally present in the blood and immunoglobulins (antibodies) which are essential for the active immune system.
Conclusion
Carbohydrates are a vast class of biomolecules who’s complexity and diversity of function rival that of proteins. Their size can range from relatively simple monosaccharides to sprawling complexes of polysaccharides. They have a unique combination of structural stability and high potential energy which makes them ideal among macromolecules for fueling metabolism, but they also have many other functions including structure and cell signaling. We do carbohydrates a disservice by only thinking of them as pasta and potatoes. They are a unique and highly diverse family of molecules essential for all life on earth.