Functional Groups in Organic Chemistry
To be successful in an organic chemistry course one needs to learn how to recognize certain patterns in molecular structure and reactions. One of those basic patterns is the recognition of the functional groups. We define a functional group as a specific combination of atoms that has a unique set of chemical properties. Thus, recognizing functional groups in a molecule will get you one step closer to identifying possible reactions and transformations you may have for that molecule.
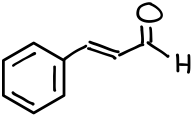
Let’s look at an example above. This is a structure of trans-cinnamic aldehyde. As the name suggests, we have an aldehyde functional group in it. However, in addition to the aldehyde functional group we also have an alkene and an aromatic compound (arene) as well.

Importantly though, not every single part of the molecule must be some sort of a functional group. So, what are the most common functional groups out there?
Hydrocarbons
We’ll start with an overview of simple hydrocarbons. Those are the functional groups consisting of only carbons and hydrogens.
Alkanes
While alkanes are not technically a functional group as there’s nothing unique to them and they don’t really have much of any chemistry associated with them, they are a backbone of organic molecules. Thus, I’m going to mention them here.
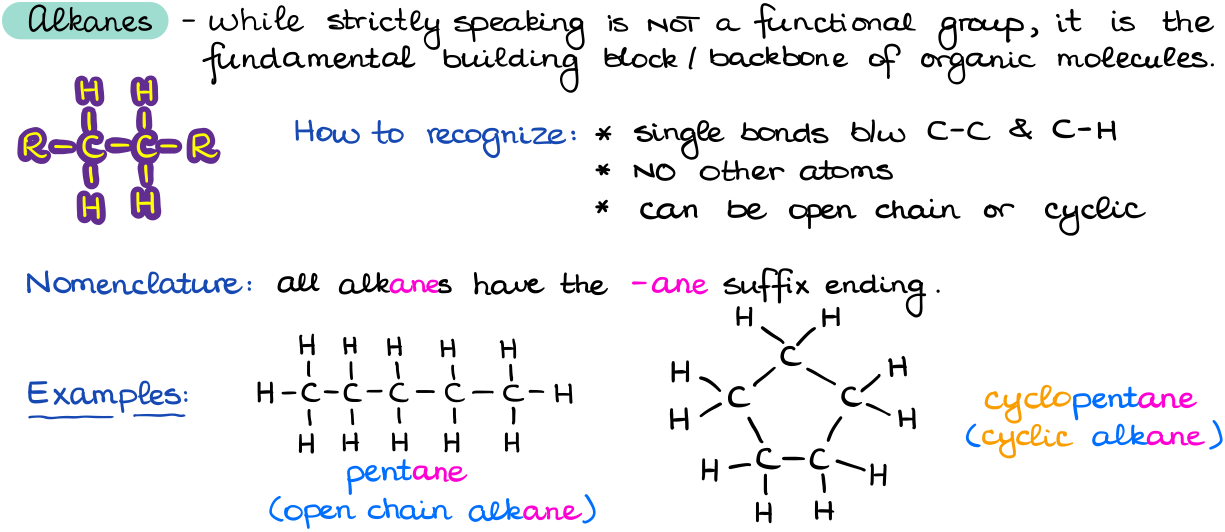
Alkanes consist of single C-C and C-H bonds, they don’t have any other heteroatoms, and they can be either open chain or cyclic. When we don’t have any other functional groups in the molecule, we use the ending -ane to signify that this is an alkane. Typical reactions of alkanes are either a simple combustion which is not very useful unless you’re trying to warm up your house or run your car, and radical halogenation.
Alkenes
The important characteristic of alkenes is the presence of a carbon-carbon double bond C=C. Just like alkanes, alkenes can be both open chain and cyclic.
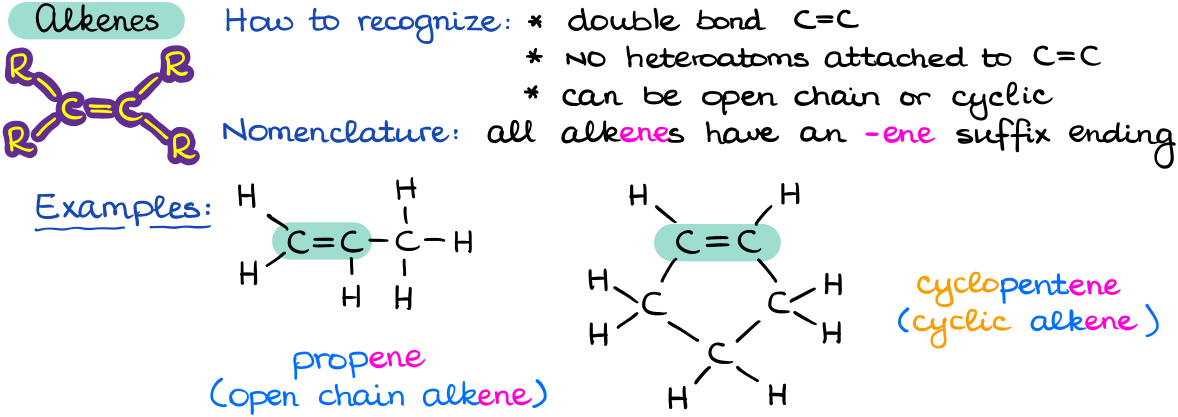
As alkenes are a functional group, we are going to signify that by changing the -ane ending to an -ene ending. Due to the rigidity of the double bond, alkenes have a lot of stereochemical properties that we are going to discuss later in this course. Additionally, the presence of the double bond gives us a lot of opportunities for functionalization of our double bond into other functional groups. The typical reaction of alkenes is the electrophilic addition to a double bond. However, we are going to see more different reactions of alkenes later in the course. So, this is one of those functional groups that we are going to be coming back to from time to time.
Alkynes
Alkynes contain a carbon-carbon triple bond.
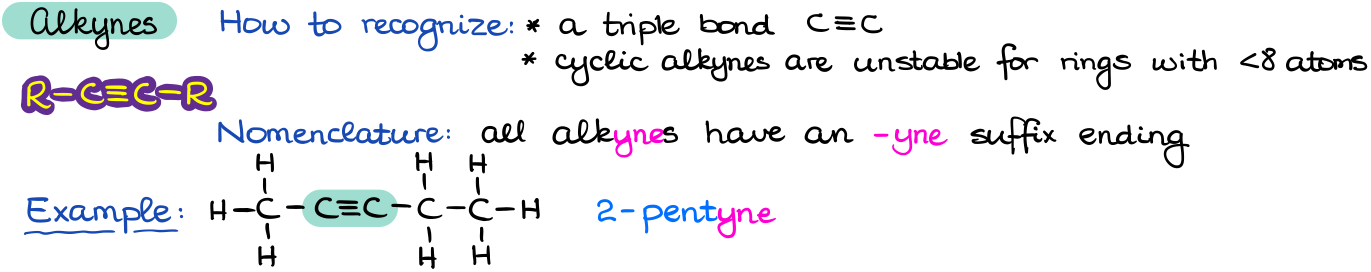
Most alkynes that you are going to encounter within the scope of this course are going to be an open-chain style molecules. Nonetheless, cyclic alkynes also do exist. The problem with cyclic alkynes and the reason for their rarity is the fact that the carbons of the triple bond are sp-hybridized which means they have a 180° bond angle. This gives a huge amount of angular strain in the molecule making cyclic alkynes very unstable. This might not make much sense now, but we’ll get back to it later when we start talking about various aspects of the structure and bonding in organic molecules.
Similar to alkenes, the nomenclature of alkynes involves the change of the –ane ending of an alkane functional group with an –yne ending for the alkyne. The most typical reaction of alkynes just like alkenes is the electrophilic addition to a π-bond.
Arenes
Arenes are also commonly called aromatic compounds and they make up a huge family of molecules. For the purposes of this discussion, we’re only going to focus on one of them—the most important one: benzene.
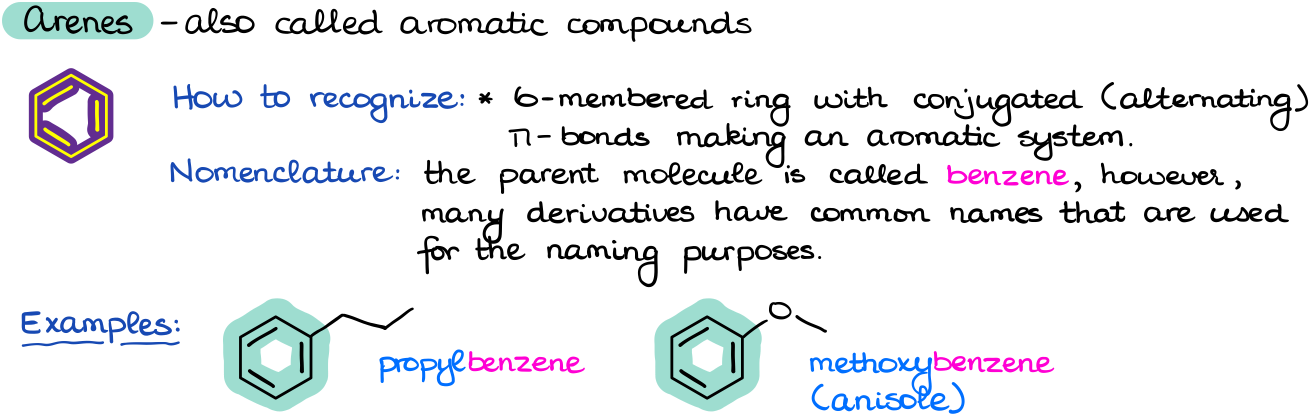
We typically draw benzene and its derivatives as a six-membered ring with alternating double π-bonds and single bonds. However, this is not quite correct and in reality there are no actual double bonds in benzene and this structure (which is, by the way, called a Kekulé structure) is merely a convenient representation.
Since aromatic compounds or arenes are a large family of compounds and cannot be represented by a single functional group structure, some instructors insist on calling the benzene by its name rather than calling it an aromatic compound. Make sure you pay attention to how your instructor likes to call it, so you don’t lose any points on exam! The typical reaction of benzenes (and aromatic compounds in general) is an electrophilic aromatic substitution.
Simple Functional Groups
Simple functional groups are typically small entities and contain just one heteroatom such as N, O, Halogens, S, etc.
Haloalkanes
Haloalkanes or alkyl halides (common name) is technically not a functional group although many instructors choose to classify it as such. When we see a halogen (F, Cl, Br, or I) attached to a carbon, you’re most likely looking at an alkyl halide.
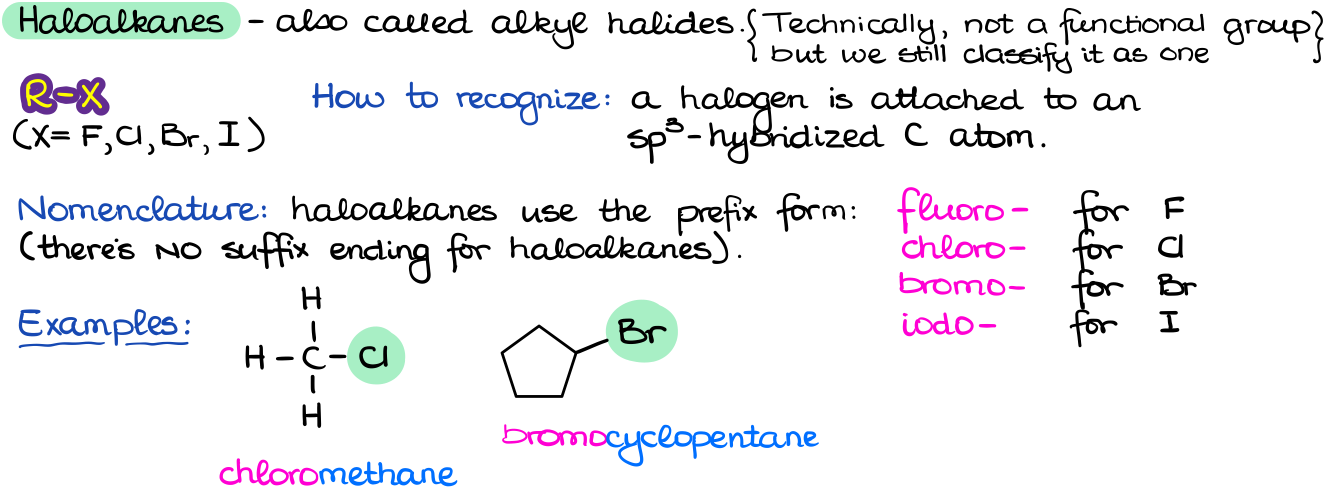
As alkyl halides are not a “real” functional group, they don’t have a special suffix that we would use at the end of the molecule name. Thus, we are always going to use the corresponding prefix form (F = fluoro, Cl = chloro, Br = bromo, and I = iodo). Alkyl halides, along with many other functional groups, typically participate in various substitution and elimination reactions.
Alcohols
Alcohols are one of the central functional groups in organic chemistry. Not only alcohols are extremely important for biochemical processes, they also have very rich chemical properties making them a versatile and essential synthetic targets as well.
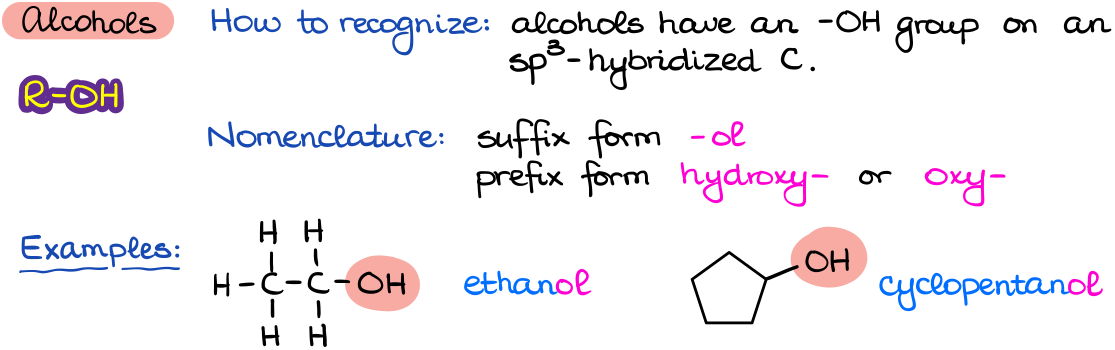
When naming alcohols, we are going to add an –ol ending to the parent (principal chain) name to signify it’s an alcohol. We may also need to specify the position of the -OH group in the molecule depending on the molecular structure.
Ethers
Ethers are quite common in nature but not a very chemically diverse functional group.
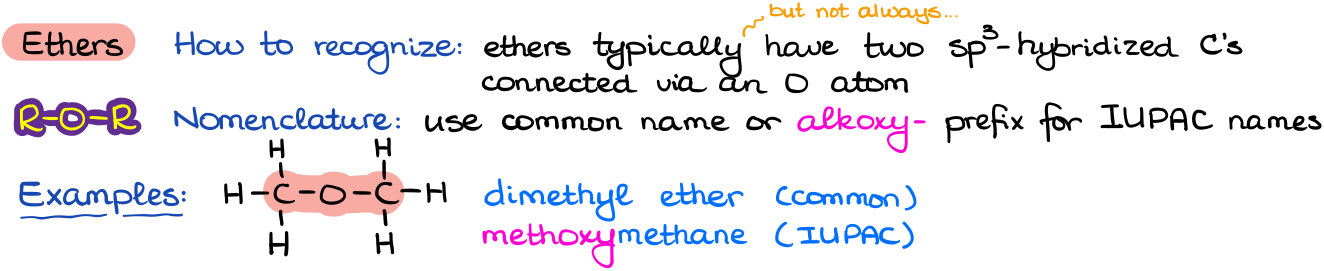
Ethers do not have their own ending or a suffix and are often called using the common names. However, the IUPAC names for ethers often show up on exams and can be somewhat tricky to construct as the would use some complex nomenclature rules. Ethers can be either open chain or cyclic molecules.
Epoxides
Epoxides are a special type of a cyclic ether. While technically they are ethers, they do in fact have unique chemical properties that other ethers lack. This makes them a separate functional group.

Due to the ring strain in an epoxide, they tend to open quite easily by various nucleophiles. This ability and easy synthesis of epoxides from alkenes makes them important synthetic intermediates yielding various alcohols. And as I have already mentioned a moment ago, alcohols rock when it comes to various functional group transformations.
The nomenclature of epoxides is quite complex. We can name them as heterocycles using “oxirane” as the parent/principal name, or we can name them as a substituent on a longer chain. Either one should be fine on the exam unless your instructor specifically requires you to use one type of the names for them.
Aldehydes
Aldehydes are common in nature and important in synthesis. This functional group can be easily recognized by the presence of the C=O bond on a carbon that is also attached to an H. The simplest aldehyde—formaldehyde—has two H’s attached to C=O. The rest of them only have one H.
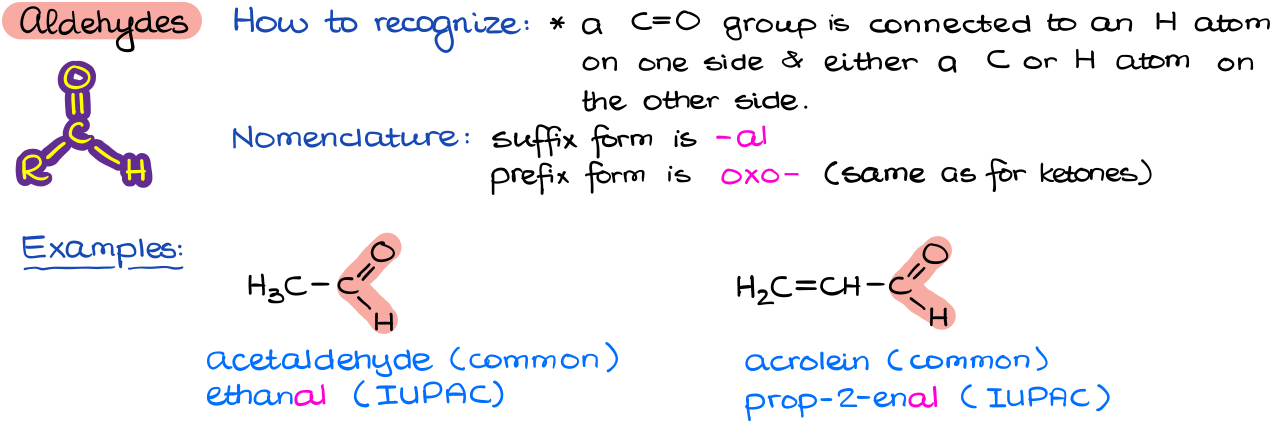
Nomenclature of aldehydes is pretty straightforward as we just have to add an ending –al to a molecule that contains an aldehyde functional group. The prefix form of an aldehyde is oxo-, which is the same as for ketones.
Ketones
Ketones are very similar to aldehydes in both look and function. They also have a C=O double bond (which we call a carbonyl). However, in the case of ketones the carbon of a carbonyl is connected to two other carbons and there are no direct carbon-hydrogen bonds connecting to a carbonyl. This might seem like an insignificant difference. However, the presence of the hydrogen in an aldehyde gives them unique chemical properties which we do not see in ketones. Thus, this makes aldehydes and ketones different functional groups.
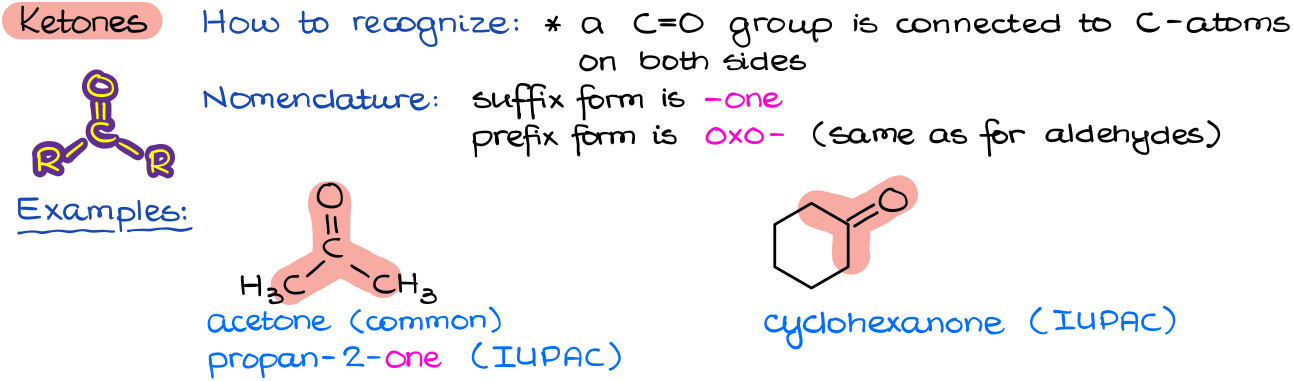
Because in a ketone a carbonyl is connected to two other carbons, we can easily have a cyclic ketone where the carbonyl is a part of a cycle itself. Also, from the perspective of nomenclature aldehydes and ketones are extremely similar to each other. The main difference is that in the case of ketones the ending is going to be –one. The prefix form, however, is the same for both: oxo-.
Both aldehydes and ketones have a very diverse chemistry. Typical reactions of aldehydes and ketones are various addition reactions to a carbonyl.
Thiols
Thiol is the simplest sulfur-containing functional group you are going to see in your course. Thiols are extremely easy to recognize as they contain an -SH group connected to a carbon atom.
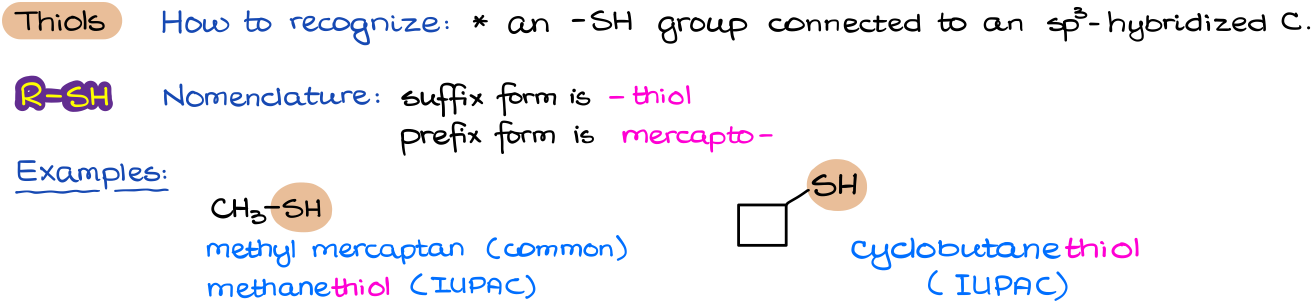
In terms of nomenclature, thiols are very similar to alcohols. To signify that we have a thiol functional group we are just going to add the ending –thiol to our molecule’s name. There are however common names associated with thiols, which you are probably going to see in your course. The most common name for thiols is mercaptan. This is an outdated and an archaic name, however it is still commonly used so it’s a good idea to remember it.
Sulfides
Sulfide is another simple sulfur-containing functional group. Unlike in thiols however, in sulfides sulfur is connected to two other carbons.

When it comes to nomenclature of sulfides, it is rather like ethers but a little bit trickier. We can use common names, or we can use two different styles of systematic names (IUPAC names). The first style of a systematic name for sulfides will use alkylsulfonyl– prefix. This is the currently preferred IUPAC name for it. However, you can still see an older and commonly used another prefix which is alkylthio-. I’m not sure when exactly that change was introduced, but I see both versions in current textbooks so it’s a good idea to know both just in case your instructor has a preference.
While there are other sulfur-containing functional groups in organic chemistry, the thiols and sulfides are among the most common ones that you are going to be required to know. The rest of them are less common and, in my experience, they do not typically show up on a test. You can look at the bottom of this page for more example of sulfur-containing functional groups.
Amines
Amines are the simplest nitrogen containing functional group. Typically, an amine is going to have a nitrogen atom connected to up to three carbon atoms and the rest are going to be hydrogens.
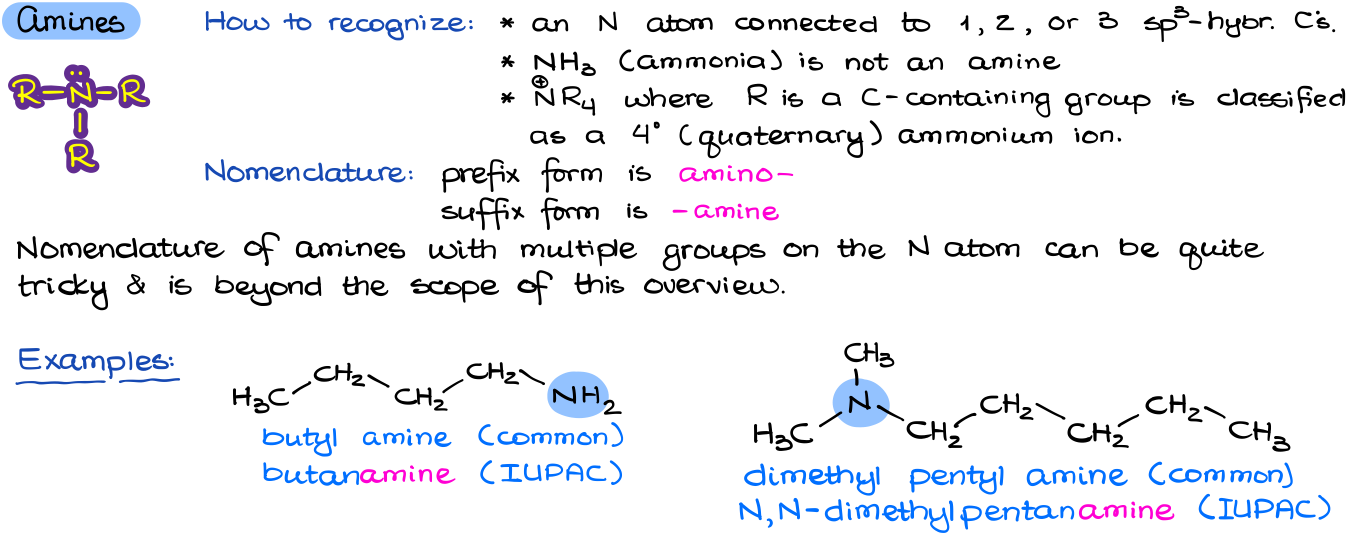
Nomenclature of amines can be quite complex. As with many cases before, we can use the common names, or we can use the systematic (IUPAC) rules. The common names are constructed by listing all the alkyl groups in an alphabetical order and then adding a word “amine”. The IUPAC names are constructed by adding the ending –amine to a parent molecule. If we have multiple groups on the nitrogen atom, we use the longest chain as the parent/principal chain and then say the rest are substituents using the N locant instead of numbers like in the example above.
In addition to complex nomenclature, amines have a very diverse chemistry. Typical reactions of amines are acid-base chemistry, various substitutions, and complex transformations. Due to the extremely diverse chemistry of amines, you’re likely to only see them much later in the course.
Nitriles
While technically nitriles are classified together with carboxylic acid derivatives, this functional group is unique enough, so I prefer to separate it out on its own.
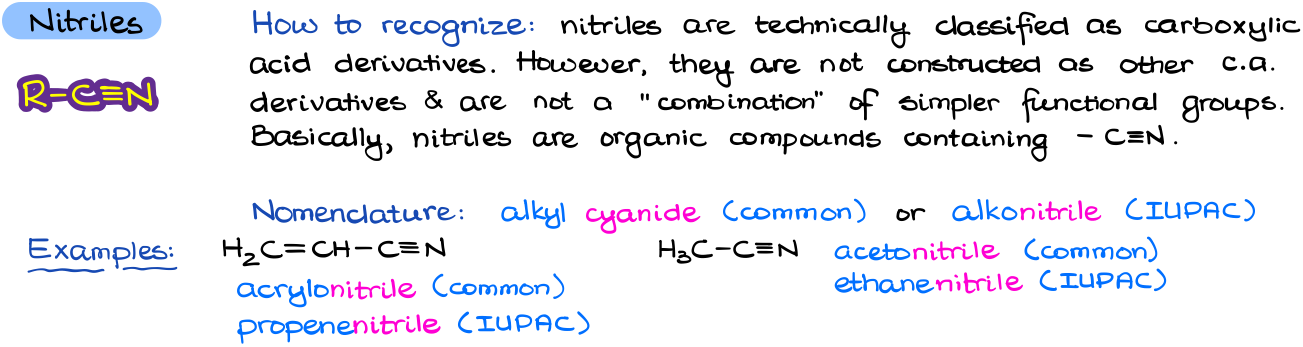
A nitrile, sometimes called a cyanide, is extremely easy to recognize: it’s a -C≡N group attached to another carbon. As they are not constructed as other carboxylic acid derivatives and do not look like a combination of other simpler functional groups, I separate them into their own category. To name a nitrile, we simply add an ending –nitrile to the end of our parent name.
Complex Functional Groups
Complex functional groups are, essentially, a combination of simpler functional groups. However, the combination of structural elements from simpler functional groups going to give new and unique chemical properties that we do not see for the individual pieces that make up the complex functional group. Important: when you’re deciding whether to go with a complex functional group or a combination of simple functional groups, always go with a more complex one.
While there are many different complex functional groups, the most common that we are going to see within the scope of a typical sophomore organic chemistry is carboxylic acids and their derivatives. All carboxylic acid derivatives, except for the nitriles, are similar in structure and share same structural features.
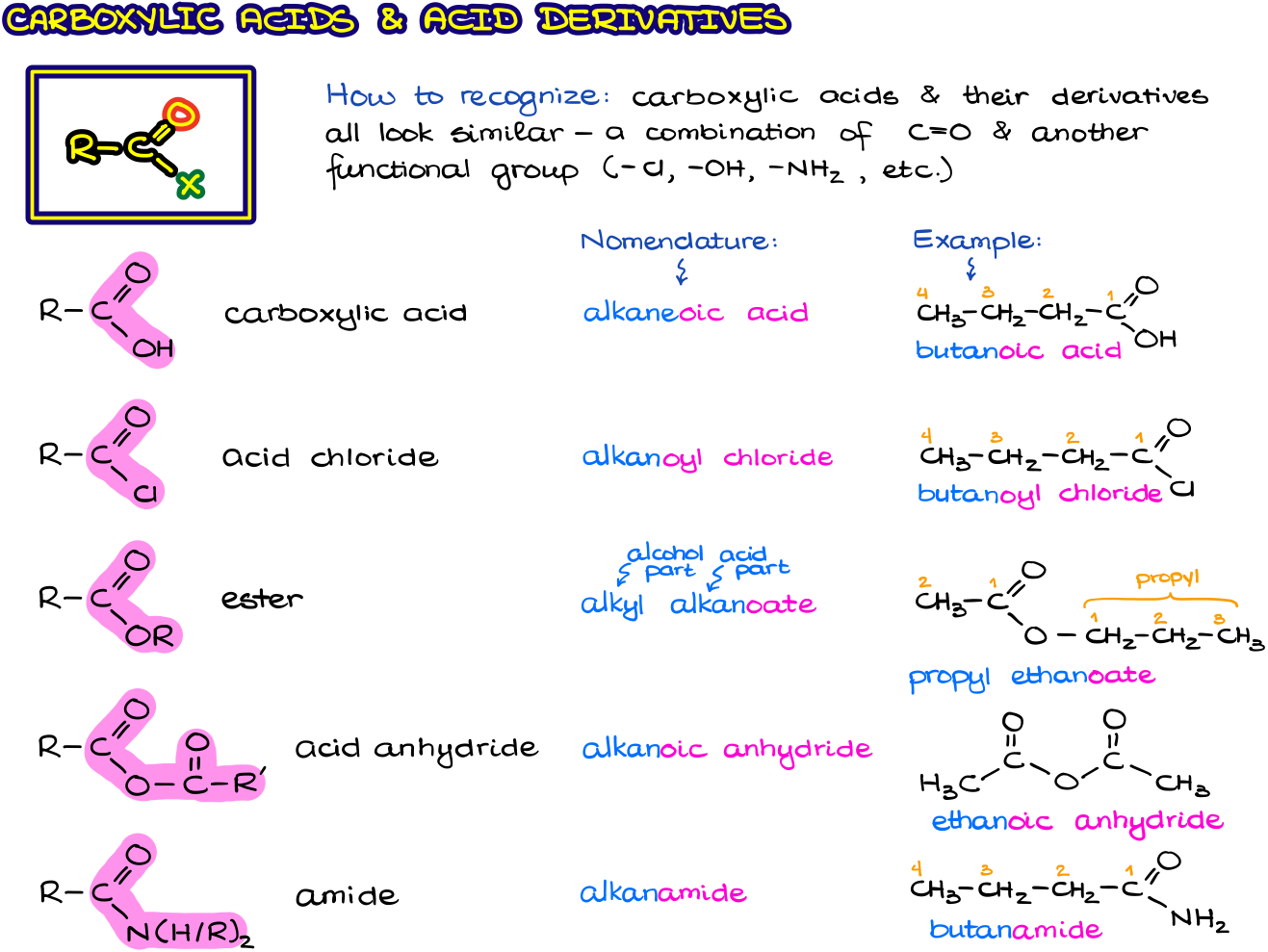
As you can see from the figure above, carboxylic acids and their derivatives all have a C=O bond in common which is connected to another group. The presence of another group next to a carbonyl adds unique chemical properties to each carboxylic acid derivative making them all into separate yet related functional groups. The most common type of a reaction you’re going to see for the carboxylic acid derivatives is various types of acyl substitution.
When it comes to the nomenclature of carboxylic acids and their derivatives, each functional group has its own naming convention making the nomenclature of carboxylic acids one of the most intricate among any other fundamental functional group we’ll see in this course.
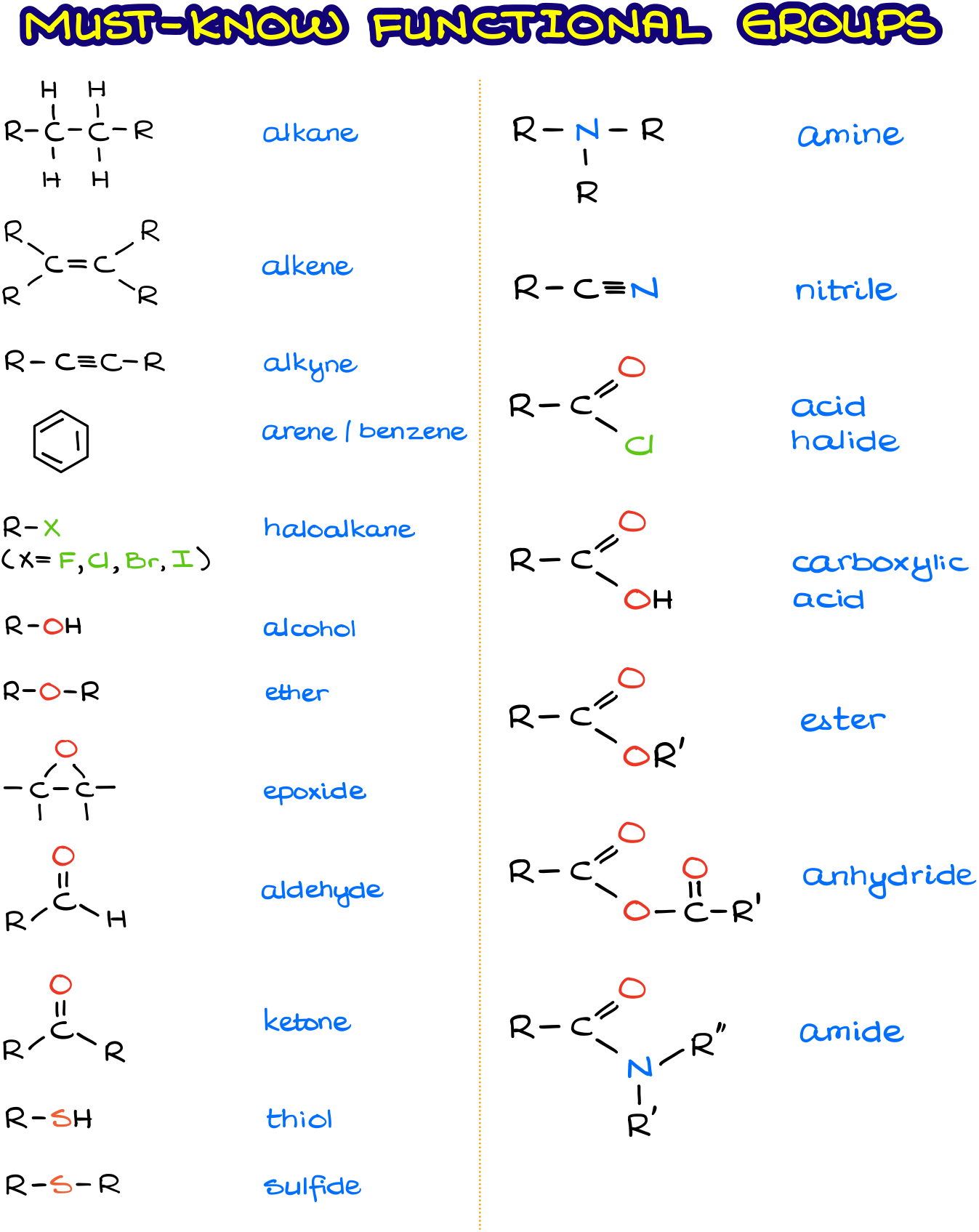
Other Important Functional Groups
There are many functional groups in organic chemistry. The exhaustive list includes over one hundred various types. Of course, you neither need to know them all nor you’re going to be tested on them all. It is a good idea though to familiarize yourself with some of the other less common functional groups you’re bound to encounter in your course.
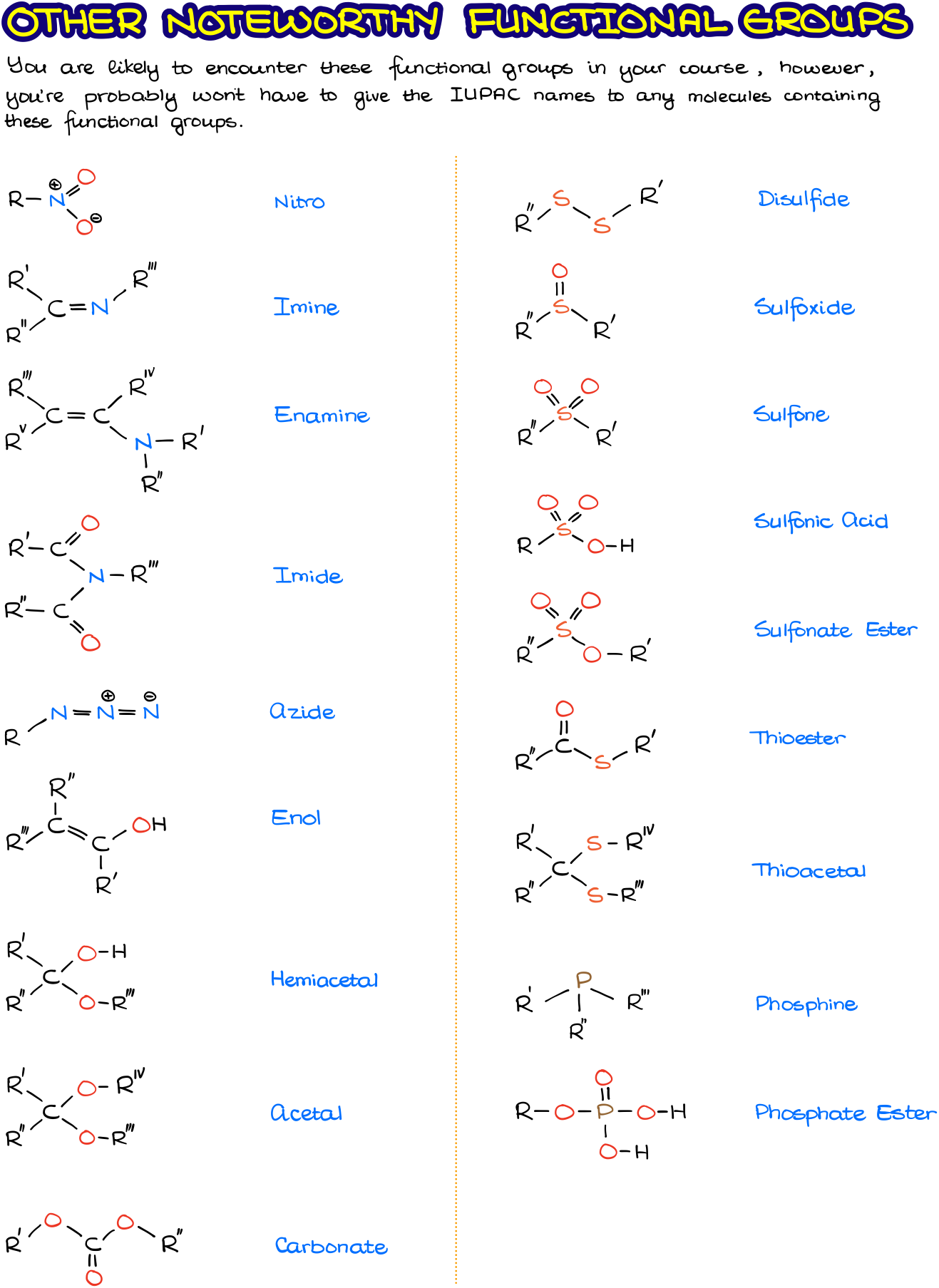
The figure above shows other commonly seen functional groups. In my experience, you’re not going to have to give an IUPAC names to molecules containing those groups.

Very helpful, thanks a lot, keep up the good work 👍
Thankyou so much for your efforts ❤️
It helps me a lot for practice questions….
Thank you! It has been easy to follow and it has allowed me to condense the information in my head, to memorize.
You’re very welcome, Julian. I’m glad this page helped you.
Excellent delivered Sir
thank you sir it was very helpful
I’m glad it helped.